Inside the dark genome
The dark genome — the long stretches of DNA that do not encode for traditional proteins — is primarily made up of repetitive elements, or repeats.
These repeats consist of different families, including LINEs (long interspersed elements; 21%), SINES (short interspersed elements; 15%), which include the Alu elements, and HERVs (human endogenous retroviruses, 9%). Other repeats range from small, simple sequence repeats to large arrays of tandem repeats at the center and ends of chromosomes.
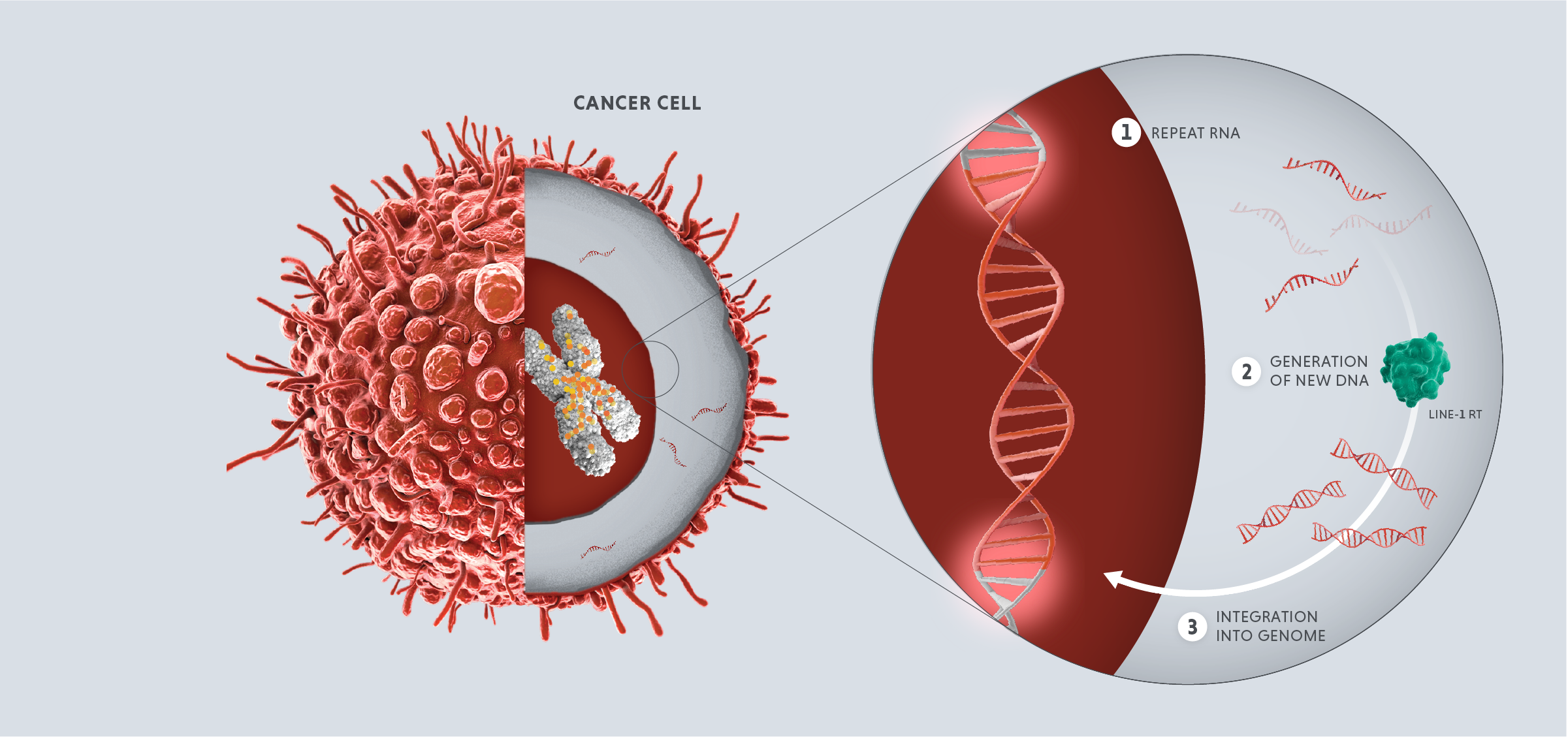
Repeats in the first category, the LINE element retrotransposons or “jumping genes,” are especially powerful. These are complex mobile elements that can make copies that transpose into new locations in the genome. These activities can alter the structure of our chromosomes and are drivers of the genetic diversity important for evolution, but because these activities are very disruptive, they are tightly controlled.









Repeats and disease
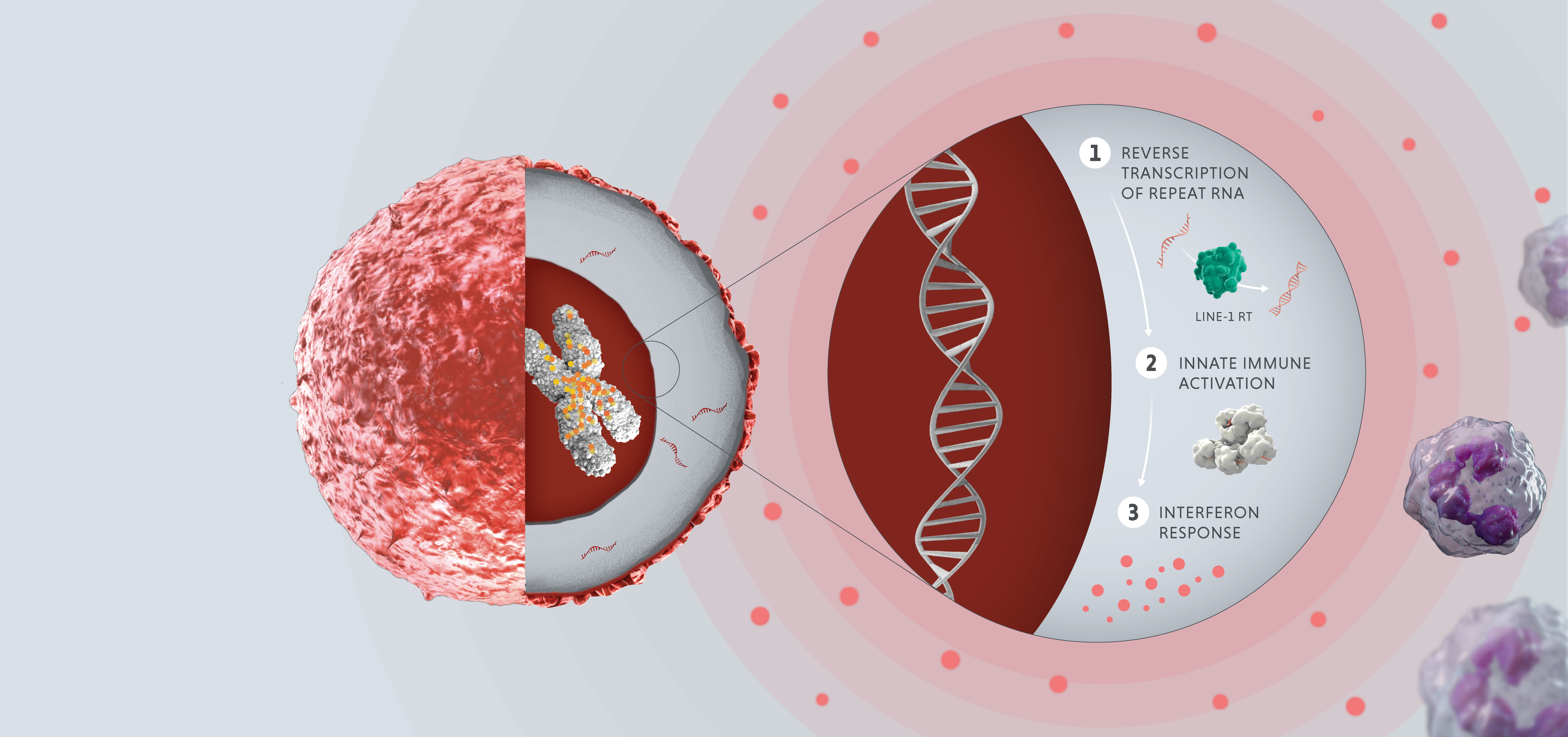
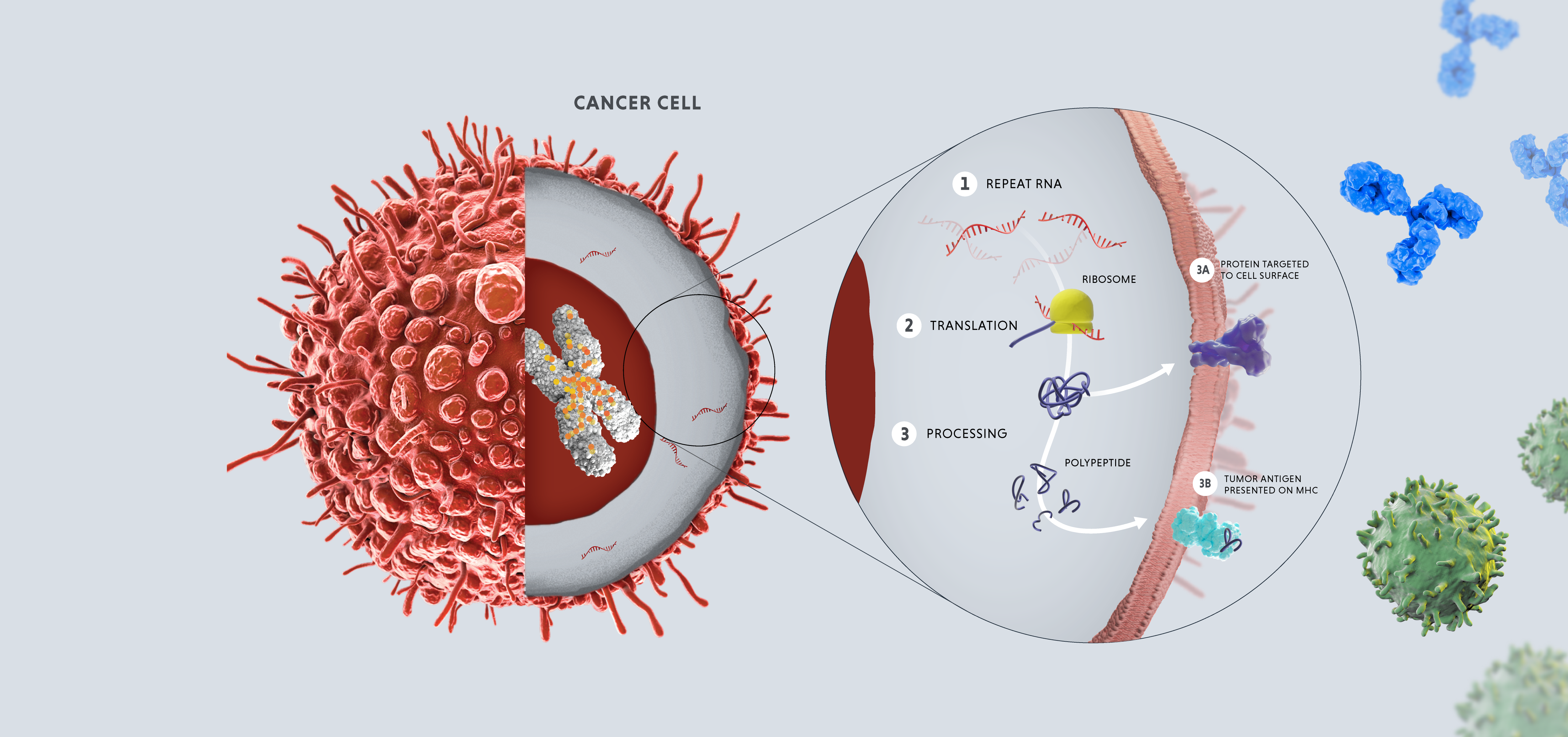
In healthy adult cells, repeats are dormant. However, in cases of disease or cellular stress, repeats can switch on. Activated repeats can be transcribed into RNA and translated into proteins, which can have downstream effects that promote disease, such as aggravating our immune system and helping tumors thrive and proliferate.
The three disease-relevant consequences of repeat activation are viral mimicry, genomic instability and expression of tumor antigens.
Explore the scientific literature
It’s only in the last decade or so that scientists have had the tools to shine light on the dark genome.
Here are some important publications for understanding the role of repeats in disease – both foundational papers that informed and inspired us when we first began to explore this field, as well as recent publications from our group and others in the community.